Vaccine storage and administration tips
Feedback from practices demonstrate that many issues arising in respect to vaccination can be minimised through optimising handling and storage. We have prepared the below guidance from practice feedback to help address some common issues regarding pet vaccines storage and administration.
-
Incorrect administration of live Bordetella vaccines
Annually, MSD Animal Health receives reports where Nobivac® KC is mistakenly administered subcutaneously. The consequences of inappropriate vaccination of a live Bordetella bacterin subcutaneously may include local and/or systemic infection which may, very rarely, prove serious. Whilst prompt antimicrobial therapy with a suitable antibiotic is often effective at avoiding more serious consequences, it is imperative that all steps are taken to prevent this avoidable accident in the first place.
We would advise the following steps should be considered to reduce the chance of mistakes:
- Ensure you are familiar with the current label information for the vaccines you administer. To make certain you act on the most up to date advice please consult the online NOAH compendium at www.noahcompendium.co.uk and/or download and consult the NOAH compendium app from the App store or Google Play store for your phone.
- Discuss the vaccines’ necessary precautions and advice with clients immediately prior to administration.
- Where more than one vaccine is administered concurrently reconstitute and draw each vaccine up separately just before their immediate use, so that syringes cannot be confused.
- Apply the nozzle to the syringe containing live Bordetella vaccine as soon as it is drawn up following reconstitution to further avoid confusion. Remember to keep your nozzle supply stocked up to avoid running out!
- Consider creating a routine of administering the injectable vaccines before intranasal vaccines to ensure a consistent approach.
- Finally, if the worst does happen and vaccines are administered erroneously, whether or not an adverse reaction is suspected, please ensure that you report the error promptly, preferably to the MA holder, ourselves in this example, who can provide appropriate advice and support, or indeed alternatively to the regulatory authority via submission of a filled out SARS form. Irrespective of route please be assured such cases are logged within the pharmacovigilance database.
Note that incorrect administration and adverse event reports for MSD products used in the UK can be reported online by practices 24 hours a day on our online form.
-
Administration of vaccines at ambient temperatures
Vaccines are more comfortable when administered at ambient rather than chilled temperatures.
The datasheets for some injectable Nobivac® vaccines e.g. Nobivac L4 specify vaccine administration at room or ambient temperatures. Professional guidelines suggest, specifically for feline vaccines, that administration at ambient temperatures constitutes better practice.
For intranasal vaccines, such as Nobivac KC, allowing the reconstituted product to warm outside refrigeration improves comfort of and acceptance of administration intranasally. Consider the following tips:
1. Nobivac solvent vials do not need to be refridgerated (store away from light and below 25 ̊C, do not freeze). If stored at room temperature this immediately helps the temperature of reconstituted vaccine.
2. Ideally, take out the vaccines you plan to use out of the fridge or chiller unit ahead of the consultation. Vaccines removed from the fridge prior to a consultation period will remain stable prior to reconstitution for the few hours needed.
3. Following reconstitution you must use the vaccine immediately or at least within the maximum period specified on the product label.
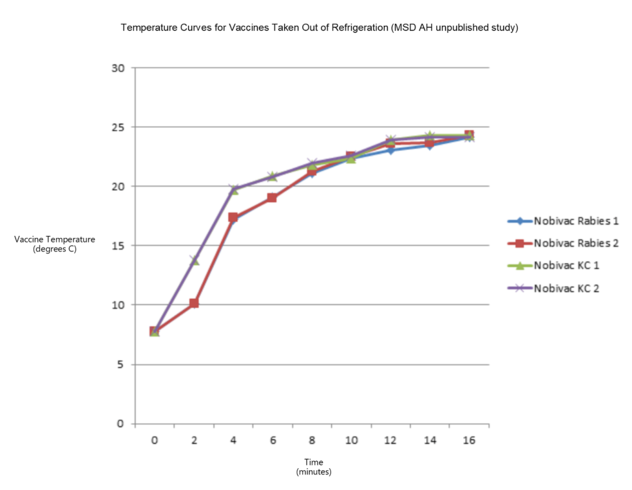
4. Should chilled solvent be used to reconstitute vaccine then it will take a number of minutes to reach room temperature. The graph below indicates the speed of warming of a 0.5ml vaccine (e.g. Nobivac KC), and a 1ml vaccine (e.g. Nobivac Rabies) under ambient conditions. Although it can take as long as 15-16 minutes to reach ambient temperature for a 1ml vaccine and 12-14 minutes for a 0.5ml vaccine, you will note that most of the warming takes place within the first 4 minutes outside refrigeration.
-
Easier administration of intranasal Nobivac® KC to dogs
Here are some tips that can make using Nobivac KC more straightforward, avoiding some of the pitfalls that can occur:
1. For improved comfort and acceptance, keep the Nobivac KC solvent out of the fridge or Nobivac Chiller Unit. Allow the reconstituted product to warm to room temperature before administration.
2. Inform your client that transient signs of sneezing maybe expected. Discuss the need for vaccination for Canine Infectious Respiratory Disease (CIRD) and recommend vaccination for at risk dogs and puppies. Ideally time vaccination at the annual booster.
3. Gentle restraint can be key to success. A soft wrap-around muzzle may sometimes be needed. An extra staff member can act as a backstop. Dogs with very short blunt noses may also benefit from a towel to help stabilise the head!
4. Administer injectable vaccine before giving Nobivac KC. Avoid approaching from the front. Covering the eyes and lifting the head can be helpful. Resting the hand holding the syringe on the side of the dog’s face may make it easier for the syringe nozzle to stay aligned in case of unexpected sharp movements.
5. If possible, try to trickle the vaccine into the nose rather than squirting it. Hold the syringe perpendicular to the long axis of the nose elevated to 45 degrees or more.
-
Vaccine storage best practice
Vaccines are supplied via a cold chain and should be stored within the practice premises (with few exceptions) under refrigeration- typically between 2 and 8 °C. Maintaining correct refrigeration conditions is required to assure that vaccine quality and usability can be maintained throughout the on-label shelf-life of the product and therefore it is vital for the vaccine supply chain and end users to regularly monitor and record fridge temperatures. These days, this can be best achieved by the use of USB thermometers.
In terms of vaccine storage, pharmaceutical fridges and bespoke chiller units have a number of advantages which minimise the chance of significant unwanted temperature deviations.
A number of issues have been identified with practice storage of vaccines, and these include inadvertent freezing of vaccines (which could potentially impact vaccine quality, safety and efficacy) where product has been kept in a part of the fridge in contact with the condenser unit. More commonly, temperatures can fluctuate upwards when fridges and chillers are not maintained, doors are left open or indeed fridges are turned off accidentally or involved in a power cut.
Deviation from recommended storage conditions necessitates the vaccine products involved to be removed from use and destroyed given that the long-term quality and shelf-life of the affected products cannot then be guaranteed. In case of doubt or if you have further questions related to this issue and MSD Animal Health vaccines please contact the Technical Product Support team via vet-support.uk@msd.com or by calling 01908 685685 (option 1) during normal office hours.
Nobivac® KC contains live Bordetella bronchiseptica bacteria (strain B-C2) and canine parainfluenza virus (strain Cornell). POM-V.
Nobivac® L4 contains inactivated Leptospira strains: L. interrogans serogroup Canicola serovar Portland-vere (strain Ca-12-000), L. interrogans serogroup Icterohaemorrhagiae serovar Copenhageni (strain Ic-02-001), L. interrogans serogroup Australis serovar Bratislava (strain As-05-073) and L. kirschneri serogroup Grippotyphosa serovar Dadas (strain Gr-01-005). POM-V.
Nobivac® Rabies contains inactivated Rabies virus strain Pasteur RIV. POM-V.
Nobivac® Solvent contains sterile phosphate buffered water. POM-V
Further information is available from the SPC, datasheet or package leaflet.
Advice should be sought from the medicine prescriber.
Prescription decisions are for the person issuing the prescription alone.
Use Medicines Responsibly.
MSD Animal Health UK Limited, Walton Manor, Walton, Milton Keynes, MK7 7AJ, UK
Registered in England & Wales no. 946942